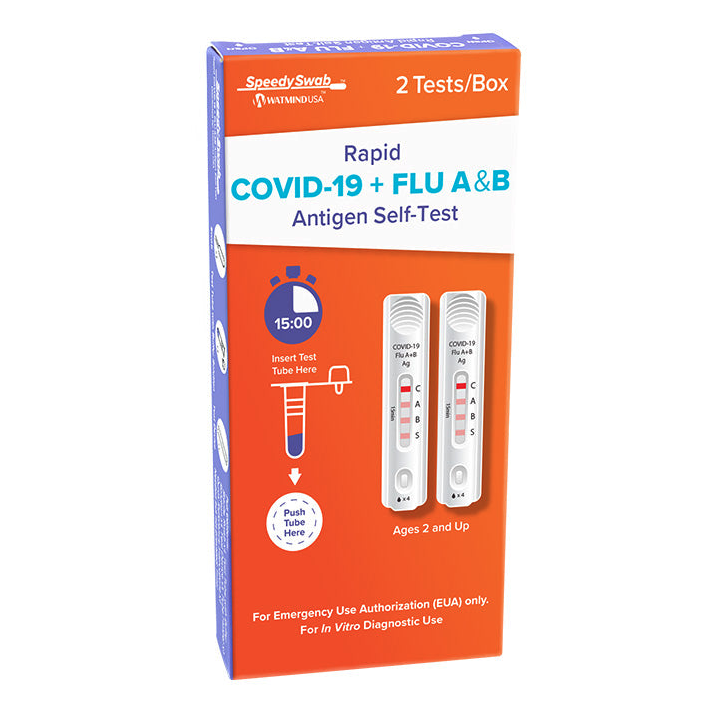
SpeedySwab™ Rapid Antigen Self-Test, COVID-19 + Flu A&B, 2 Tests/Box
Fast, Reliable At-Home Testing for COVID-19 & Flu
The SpeedySwab™ Rapid COVID-19 + Flu A&B Antigen Self-Test is a simple and convenient at-home test designed to quickly and accurately differentiate between COVID-19 and influenza A & B. This test provides rapid results in just 15 minutes, helping individuals make informed health decisions.
Product Highlights
Dual Detection: Differentiates between COVID-19, Flu A, and Flu B with a single test.
Fast & Reliable: Results in just 15 minutes for quick action.
Easy-to-Use: Simple anterior nasal swab collection, with self-collection for ages 14+ and adult-assisted collection for ages 2+.
At-Home Convenience: No prescription or lab visit required.
Up to 18-Month Shelf Life: Ensures availability when needed.
Who Can Use the SpeedySwab™ Self-Test?
Individuals aged two and older with symptoms of a respiratory infection consistent with COVID-19 or the flu.
Ages 14+ can self-collect their sample.
Ages 2+ require an adult to collect the sample.
This test is authorized for non-prescription home use in individuals with COVID-19 symptoms that began within the last 5 days. For the most accurate results, test twice over three days, with at least 48 hours between tests.
Frequently Asked Questions
-
SpeedySwab™ is a lateral flow antigen test that detects and differentiates COVID-19, Influenza A, and Influenza B proteins using a simple nasal swab sample. The test provides results in just 15 minutes.
-
This test is authorized for:
Individuals aged 14+ who can self-collect their nasal swab.
Children ages 2-13, with an adult collecting the sample.
Individuals experiencing symptoms of respiratory infection consistent with COVID-19 or the flu, within the first five (5) days of symptom onset.
Physicians may also consider individuals with a family history of lung cancer or elevated risk due to environmental exposure and other risk-factors. LEAP can serve as a decision aid for individuals putting off lung cancer screening due to concerns about radiation exposure and the need for repeat scans over time.
The referring physician should judge a patient’s risk in light of clinical factors following conversations with the patient. LEAP is not for those already diagnosed with lung cancer or currently undergoing treatment for lung cancer.
-
The test has been clinically validated with the following sensitivity rates:
COVID-19: 92.6%
Flu A: 82.9%
Flu B: 90%
-
The test device includes three separate result lines:
COVID-19 (SARS-CoV-2) Line
Influenza A Line
Influenza B Line
A control line ensures that the test was performed correctly. Instructions on result interpretation are included in the test kit.
-
For the most accurate results, it is recommended to test at least twice over three days, with at least 48 hours between tests.
-
Yes, the test has received Emergency Use Authorization (EUA #EUA240014) from the U.S. FDA.
-
Yes, the SpeedySwab™ Rapid COVID-19 + Flu A&B Test is available in a 25-test pack, designed for use by healthcare providers and organizations that need multiple tests. Learn more ⟩⟩